Cross-coupling by a noncanonical mechanism involving the addition of aryl halide to Cu(II)
Abstract
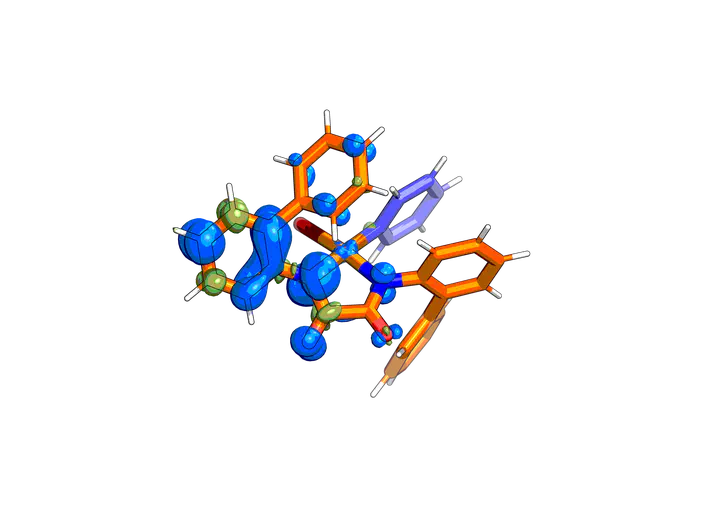
Copper complexes are widely used in the synthesis of fine chemicals and materials to catalyze couplings of heteroatom nucleophiles with aryl halides. We show that cross-couplings catalyzed by some of the most active catalysts occur by a mechanism not previously considered. Copper(II) [Cu(II)] complexes of oxalamide ligands catalyze Ullmann coupling to form the C–O bond in aryl ethers by concerted oxidative addition of an aryl halide to Cu(II) to form a high-valent species that is stabilized by radical character on the oxalamide ligand. This mechanism diverges from those involving Cu(I) and Cu(III) intermediates that have been posited for other Ullmann-type couplings. The stability of the Cu(II) state leads to high turnover numbers, >1000 for the coupling of phenoxide with aryl chloride electrophiles, as well as an ability to run the reactions in air.